One of the most prominent examples of viral escape in the recent years has been SARS-CoV-2 of which new variants have acquired more and more escape mutations that led to a significantly hampered detection and neutralization of the virus by the humoral immune system and monoclonal antibodies. However, while viruses like SARS-CoV-2 that result in an acute infection only rarely develop escape mutations in most patients, chronically infecting viruses like HIV-1 can develop numerous escape mutations in a single patient over the years which leads to a co-evolution of the immune system and the virus. In very few patients, this co-evolution leads to the development of broadly neutralizing antibodies (bNAbs) that can neutralize up to 100% of worldwide circulating strains. Antibodies have been ground-breaking in the therapy of autoimmune diseases and cancer and with the recent identification of new highly potent bNAbs, antibodies will also play a key role in future HIV-1 treatment and prevention strategies. HIV-1 reactive bNAbs that target different epitopes on the envelope trimer (Figure 1) have been tested in many clinical studies in recent years, where they demonstrated safe suppression of viremia, a delay of viral rebound after interruption of ART and protection of humans against sensitive strains. The characterization of the interplay between bNAbs and the HIV-1 envelope protein also significantly facilitated the design of novel HIV-1 vaccines of which several candidates are currently already being evaluated in clinical studies.
As for any drug against HIV-1, viral resistance and escape represent formidable challenges for currently available bNAbs. Effective bNAb therapies and vaccines could be hampered by de novo and pre-existing HIV-1 antibody resistances (HIVAR). RNA viruses, like HIV-1, are characterized by exceptionally high rates of spontaneous mutation. Especially the HIV-1 envelope protein (HIVenv) can rapidly evade the immune pressure mediated by neutralizing antibodies and clinical trials have shown that such mutations pre-exist in many patients and/or can develop quickly de novo during treatment with bNAbs in humans. Moreover, HIV-1 vaccines that are designed to elicit bNAbs in vaccinees can only be protective against sensitive strains. Thus, rapid testing of patients or even large cohorts for their bNAb sensitivity is crucial for future clinical use of bNAbs of bNAb-inducing vaccines (Figure 2).
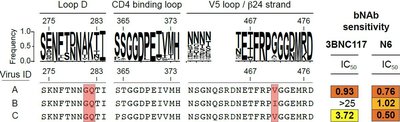
Our group aims to develop novel methods that will allow the rapid identification of HIV-1 antibody resistances (HIVAR) from replication or translationally competent proviruses in HIV-1 infected individuals (Figure 3). This will help to further characterize and understand HIVAR which will be of upmost importance for future clinical studies. As a result, our work will significantly help to improve future bNAb treatment and prevention strategies as well as HIV-1 vaccines that elicit bNAbs and has the capability to significantly improve the life of HIV-1-infected patients.